Exploring new electrode material architectures is critical to the development of large-capacity and high-rate lithium/sodium (ion) batteries. The open frame structure is not only conducive to the rapid reaction of sodium ion channel construction, the spatial distribution of the product after its structure decomposition is also conducive to the improvement of multi-electron conversion reaction activity and efficiency, the discrete distribution of the framework phase structural unit can even make the conversion reaction occur in On the molecular scale, the spatial distribution of the conversion products can be further improved, and the enthalpy through which the ions pass through the multiphase interface can be slowed down.
Recently, the research team led by Li Chilin, a researcher of the Shanghai Institute of Ceramics, Chinese Academy of Sciences, has made a series of advances in the structural synthesis design of frame electrodes for lithium/sodium batteries. Related results were published in ACS Nano, Chem. Mater, and J. Mater. Chem. A (2) and other journals.
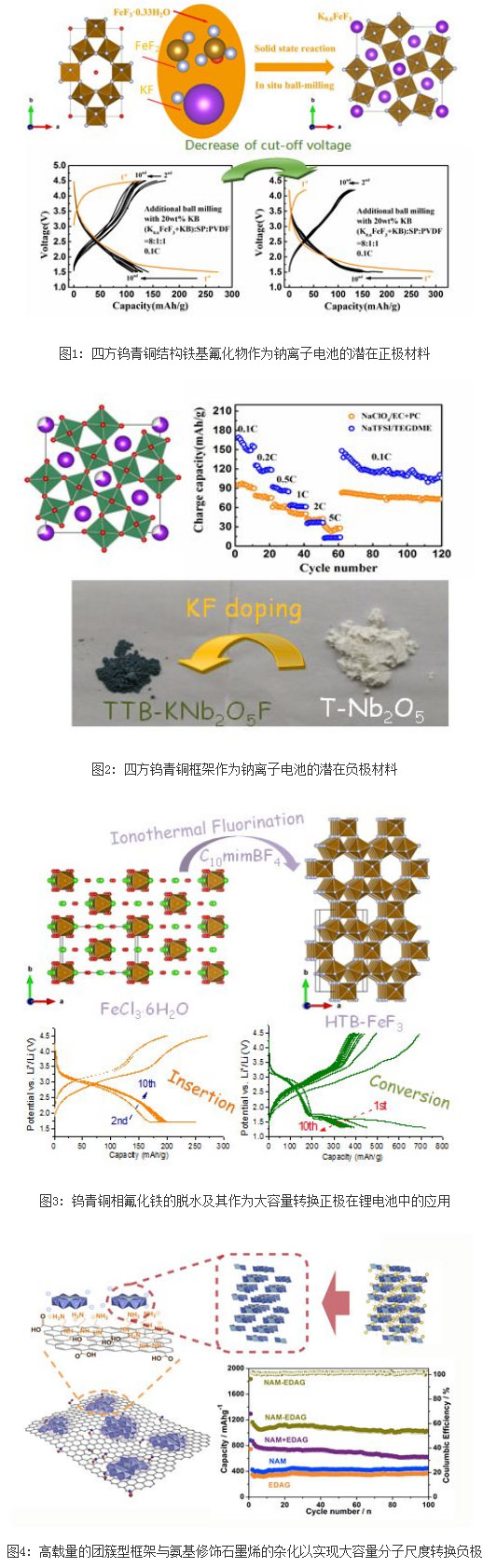
Following the first realization of hexagonal tungsten bronze (HTB, FeF3 0.33H2O) and pyrochlore phase (Pyrochlore, FeF3 0.5H2O) fluorides for sodium battery cathode materials (J. Am. Chem. Soc. 2013, 135, 11425-11428 (Chem. Mater. 2013, 25, 962-969). The team recently proposed once again the use of a tetragonal tungsten bronze (TTB) open frame structure for positive and negative electrode materials for sodium-ion batteries. The TTB phase replaces water molecules with potassium ions with better thermal stability as the channel filling, which not only realizes a more robust open frame phase, but also enables simple solid-phase synthesis and in-situ milling synthesis routes, and positive charge fillers also make it possible. Iron fluoride is in a reduced state that favors "ion batteries." The tunnel-type TTB phase can be obtained by topologically compacting the same tunnel-type HTB phase. During the period, the intrinsic Fe-F octahedral chain does not break, but their linking modes change, and the only hexagonal edge tunnel in the HTB is converted to TTB. Staggered pentagonal and quadrangular side tunnels. The pre-supporting of larger-sized potassium ions enables the sodium-bearing electrochemical behavior with similar zero stress of TTB. K0.6FeF3 achieves the first charge-out charge capacity of 125 mAh/g and the subsequent reversible sodium release capacity of 100–150 mAh/g. The thiol-based TTB phase (KNb2O5F) can be used as an embedded negative electrode material for a sodium ion battery. It can be realized by a simple KF-doped commercial Nb2O5, in which K and F exist as channel supports and coordination substituents, respectively. This strategy significantly increases the electronic and ionic conductivity of the material, showing high cyclic stability even for ceramic-like large particles without any modification. The relevant research results are published in J. Mater. Chem. A, 2016, 4, 7382-7389 (Figure 1) and Chem. Mater. 2016, 28, 3139-3147. (Figure 2).
Lithium metal batteries based on conversion reactions are receiving increasing attention due to their higher energy densities. To take full advantage of the advantages of the fluoride open frame phase in conversion reactions, it is first necessary to remove the channel crystal water. Because crystalline water molecules act to stabilise the framework of the framework by bonding in the channels, they are not easily removed, and even removal is often accompanied by densification and amorphization of the open framework phase. Following the dehydration of the Pyrochlore phase in the earlier stage (Adv. Energy Mater. 2013, 3, 113-119.), the team recently succeeded in dehydrating the HTB phase through ion-heat fluorination in a microphase-separated ionic liquid. Increasing the crystallinity of the HTB phase (reducing the channel defects) and reducing the surface coating is the two key factors for removing the channel water molecules. As a switching anode, its reversible capacity reaches 200-450 mAh/g at least 100 cycles. Compared to the water containing crystal water FeF3 0.33H2O, the capacity increased by 100 mAh/g. The relevant research results were published on J. Mater. Chem. A 2016, 4, 16166-16174 (Figure 3).
Compared with the conventional ligand compact link-type conversion electrode, the open framework phase composed of discrete structural units with conversion reaction activity facilitates the conversion reaction to occur at the molecular scale. The team used cluster-type heteropoly acid (POM) as an example, through the Al (Si)-driven polymerization, and its polyanionic groups and positively charged graphene were electrostatically hybridized to achieve the POM material in the electrolysis Low solubility in liquids and high loading in conductive networks. The Al-based POM exhibits a reversible capacity of more than 1000 mAh/g as a lithium storage anode and a long cycle of more than 1100 cycles, and can tolerate current densities up to 20 A/g. The six-electron conversion reaction occurring at the molecular scale and the spatial distribution optimization of the accompanying conversion products are the key to realize the high electrochemical activity of this cluster-type framework-opening phase. However, the sodium storage capacity is significantly less than the lithium storage capacity, verifying that the team's previously proposed "lithium/sodium-driven conversion reaction activity and efficiency are highly dependent on their reaction path changes and the resulting hybrid conductive network evolution" (J Mater. Chem. A 2015, 3, 509-514). The relevant research results were published on the ACS Nano 2016, 10, 5304-5313 (Figure 4).
The related research work has been funded and supported by the Chinese Academy of Sciences 100-person plan, the National Natural Science Foundation, and the China Postdoctoral Science Foundation.
Reactive Dyes For Printing,Popular Reactive Dyestuff,Reactive Yellow For Cotton,Reactive Orange K-Gn
ZHEJIANG E-DYE SUPPLY CHAIN MANAGEMENT CO.,LTD. , https://www.easytodyes.com