The team of Dr. Zhao Chenglong, associate researcher Lu Yaxiang, and researcher Hu Yongsheng from the Institute of Physics, Chinese Academy of Sciences/National Research Center for Condensed Matter Physics, in collaboration with Prof. Marnix Wagemaker from the Delft University of Technology in the Netherlands, and Prof. Claude Delmas from the University of Bordeaux in France, proposed a The method for predicting the configuration of sodium ion layered oxide and confirming the effectiveness of this method experimentally provides theoretical guidance for the design and preparation of low-cost, high-performance sodium ion battery layered oxide cathode materials. On November 6, the relevant research results were published online in Science on the topic of "Rational design of layered oxide materials for sodium-ion batteries" (Science).
Layered oxides have periodic layered structures and two-dimensional ion transport channels, and are a type of intercalation compound that has been studied earlier. Since 1980, lithium-ion layered oxides (LiMO2, M is one or more transition metal elements or other doping elements) have been the main cathode materials for lithium-ion batteries, which are repeated by co-edge MO6 octahedrons. In the layer structure of these layers, lithium ions are located in an oxygen octahedral coordination environment, forming an O-type (Octahedral, octahedral) stacking configuration. In the process of exploring electrode materials for sodium ion batteries, sodium ion layered oxide (NaxMO2) has become the research object. However, unlike LiMO2, which tends to form an O-type structure, sodium ions have two coordination environments with oxygen between the layers formed by the MO6 polyhedron, which can be divided into two configurations: O and P (Prismatic, triangular prism). Among them, O3 and P2 is the two more common structures in the layered cathode material of sodium ion batteries (the number represents the number of stacked layers with the least repeating unit of oxygen, such as 2 corresponds to ABBA..., 3 corresponds to ABCABC...) (Figure 1A). The layered oxides of these two structures have their own advantages when used as the cathode material of sodium ion batteries: O3 phase cathode material has a higher initial Na content, can extract more sodium ions, and has a higher capacity; P2 phase cathode material The material has a large Na layer spacing, which can increase the transmission rate of sodium ions and maintain the integrity of the layered structure, and has better rate performance and cycle performance. In order to find a positive electrode material with good sodium storage performance, the researchers synthesized a large number of O3 and P2 materials, and found that in the material preparation process, different sodium content, different transition metal element types, valence states and compositions may lead to different structures. The layered material in turn affects the electrochemical performance of the cathode material. However, in addition to physical characterization of the synthesized material to determine its specific configuration, there is currently no method to directly predict the stacked structure of layered materials and guide the design and preparation of layered oxide cathode materials.
It is an important research direction to explore the factors affecting the formation of the two configurations of sodium ion layered oxides. In 1976, Delmas et al. (Materials Research Bulletin, 1976, 11, 1483-1488) proposed to use Rouxel diagram (Journal of Solid State Chemistry, 1976, 17, 223-229) to distinguish the stacked structure of NaxMO2, indicating the sodium content and anion and cation The nature of the intermolecular bond (ionic and covalent) is an important factor in determining the layered stack structure. However, this method only considers the difference in Pauling’s electronegativity. For layered structures with the same transition metal elements but different valences (such as Na0.7MnO2 containing both Mn4+ and Mn3+) or containing multiple transition metal elements and doped The layered structure of miscellaneous elements is difficult to predict accurately.
This problem also caused the attention of the sodium-ion battery research team led by Hu Yongsheng. They found that different transition metal elements in the same valence state will obtain different structures during the material preparation process. They speculate that the ionic radius of the transition metal may be used to predict the stack structure, so the concept of equivalent radius is introduced (the equivalent radius is The weighted radius is the radius of the transition metal multiplied by the content of the transition metal). Through the analysis of the reported materials, it is found that the larger the equivalent radius, the easier it is to form the O3 phase, and vice versa, the easier it is to form the P2 phase (Qi Xingguo, Research and Industrialization of Layered Oxide Materials for Sodium Ion Battery [D]. Institute of Physics, Chinese Academy of Sciences, 2018). However, for different series of layered oxides, the critical value of the equivalent radius is not exactly the same, and the change trend of the equivalent radius can only be used to determine which stack structure is easy to form. In the process of summarizing the structure parameters of different series of layered oxides, the research team found the ratio of Na layer spacing (d(O-Na-O)) and M layer spacing (d(OMO)) of O3 and P2 structural materials There is a critical value of 1.62. A ratio higher than 1.62 usually forms a P2 phase, and a ratio below 1.62 easily forms an O3 phase. The change of the layer spacing is essentially the result of the interaction of the electrostatic attraction and electrostatic repulsion between the NaO2 layer and the MO2 layer. Increasing the sodium content can enhance the electrostatic attraction between the Na layers, making d(O-Na- O) becomes smaller to obtain the O3 phase; on the contrary, the electrostatic repulsion in the P2 phase plays a major role (Chenglong Zhao, et al., Angewandte Chemie International Edition, 2018, 57, 7056-7060). The study further recognizes the nature of the formation of O3 and P2 configurations, but it can only confirm the configuration of the material by calculating the layer spacing ratio after structural characterization, but cannot directly guide and predict the stacked structure of layered materials.
Based on this, this study introduces the variable parameter "cation potential (Φcation)", which normalizes the oxygen anion potential by normalizing the weighted average ion potential of transition metals or other doping elements in NaxMO2 and the weighted average ion potential of sodium. Describe the electron cloud density and polarization of cations, reflect the interaction between the alkali metal layer (O-Na-O) and the transition metal layer (OMO) in the layered oxide, to indicate the difference between the O3 type structure and the P2 type structure Competitive relationship. In order to distinguish O3 and P2 structures more intuitively, a large number of O3 and P2 materials that have been experimentally verified are distributed in a rectangular coordinate system with Φcation as the abscissa and ordinate, and it is found that the fitted "boundary line" can be used to distinguish O3 and P2 have two configurations, so as to obtain the "phase diagram" of O3 and P2 structure (Figure 1B). The phase diagram can be used to guide the design of layered oxide materials. For example, for a layered material with a specific Na content, to obtain a P2 structure, it can be adjusted by increasing the cation potential; the increase of the cation potential means the enhanced MO interaction, This leads to a decrease in the d(OMO) spacing and an increase in the d(O-Na-O) spacing, which is conducive to obtaining the P2 configuration. If the content of sodium ions in the sodium layer is increased, the electrostatic attraction of Na-O will increase, thereby enhancing the shielding ability to the repulsive force of transition metals, resulting in the structure changing from P2 phase to O3 phase. Due to the large selection of elements in the transition metal layer, a reasonable combination of transition metal ions can weaken the influence of sodium ion content to a certain extent. Therefore, the cation potential makes it possible to predict the P2 and O3 phases of sodium ion layered oxides. In order to further verify the actual guiding role of this method in material synthesis, Na[Li1/3Mn2/3]O2 was used as the initial composition, and sodium-rich O3-Na[Li1/3Ti1/6Mn1/2]O2 was synthesized by adjusting the cation potential (Figure 2) and high sodium P2-Na5/6[Li5/18Mn13/18]O2 (Figure 3) two pure phase layered oxide cathode materials, and show a higher sodium storage capacity. Finally, it was found that the method of regulating the structure of sodium-based layered oxides with cation potential is also applicable to layered oxides of lithium and potassium (Figure 4).
This research provides a new method for the design of layered oxide structure and confirms the effectiveness of the method with experiments, laying a scientific foundation for the design and preparation of layered oxide cathode materials for low-cost, high-performance sodium ion batteries. The research work has been awarded by the National Science Fund for Distinguished Young Scholars, the National Key R&D Program Project, the Chinese Academy of Sciences Strategic Leading Science and Technology Project (Class A), the Beijing Municipal Commission of Science and Technology Project, the Beijing Natural Science Foundation-Haidian Original Innovation Joint Fund Project and the Chinese Academy of Sciences Youth Innovation Promotion Will wait for project support.
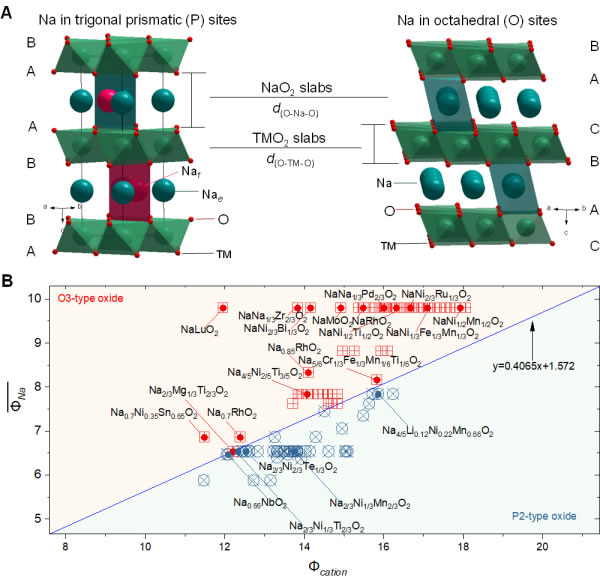
Figure 1. Cation potential and its application in sodium ion layered oxides. (A) Schematic diagram of the crystal structure of typical P2 and O3 sodium ion layered oxides; (B) P2 and O3 sodium ion layered oxides with different sodium content, different transition metal oxidation states and compositions Φcation is the abscissa, and the weighted average ion potential of sodium is the distribution diagram in the rectangular coordinate system of the ordinate
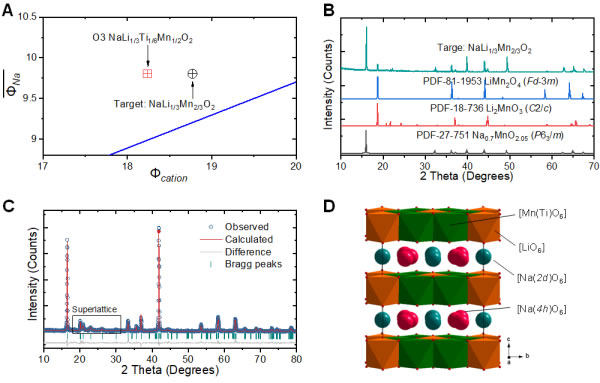
Figure 2. Design of sodium-rich O3 material based on cation potential. (A) Analysis of cation potential of Na-Li-Mn(Ti)-O; (B) XRD pattern and related standard pattern of initial composition Na[Li1/3Mn2/3]O2; (C) O3-Na[Li1/3Ti1 /6Mn1/2]O2 structure refined XRD pattern; (D) [Li1/3Ti1/6Mn1/2]O2 layer Li/Mn(Ti) ordered structure diagram
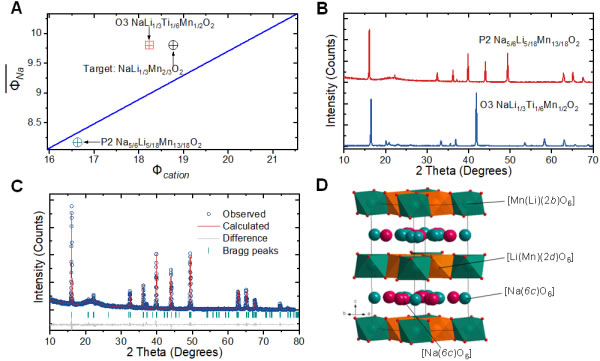
Figure 3. Design of high sodium P2 material based on cation potential. (A) Cation potential analysis of Na-Li-Mn(Ti)-O; (B) XRD of O3-Na[Li1/3Ti1/6Mn1/2]O2 and P2-Na5/6[Li5/18Mn13/18]O2 Map; (C) P2-Na5/6[Li5/18Mn13/18]O2 structure refined XRD pattern; (D) [Li5/18Mn13/18]O2 layer Li/Mn ordered arrangement structure diagram
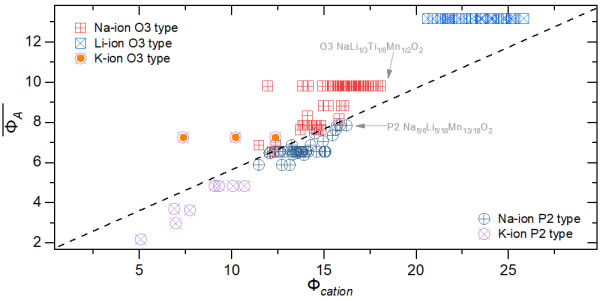
Figure 4. Summary of the reported cation potential phase diagrams of Li-/Na-/K alkali metal layered oxides
Double-Column Universal Material Tensile Test Machine
A Universal Tensile Testing Machine, also known as a universal testing machine (UTM) or a materials testing machine, is a versatile instrument used to determine the mechanical properties of materials. It is commonly used in industries such as manufacturing, construction, and research laboratories.
The machine applies tension, compression, bending, and shear forces to test the strength, elasticity, and durability of various materials such as metals, plastics, textiles, rubber, ceramics, and composites. It can measure parameters such as tensile strength, yield strength, elongation, modulus of elasticity, and fracture toughness.
A typical UTM consists of a load frame, grips or fixtures to hold the specimen, a load cell or force transducer to measure the applied force, and a displacement transducer to measure the deformation or displacement of the specimen. The machine is controlled by a computer or a control unit that allows the user to set the testing parameters and record the test data.
During a tensile test, the specimen is placed between the grips and a controlled force is applied until the specimen fractures. The machine continuously measures the applied force and the corresponding deformation or displacement of the specimen. The data is then plotted on a stress-strain curve, which provides valuable information about the material's behavior under tension.
Universal Tensile Testing Machines are crucial in quality control and material selection processes. They help manufacturers ensure that their products meet industry standards and customer requirements. They also aid researchers in developing new materials and improving existing ones.
Double-Column Universal Material Tensile Tester,Material Testing Machine,Material Fatigue Testing Machine,Universal Material Testing Machine
Dongguan Best Instrument Technology Co., Ltd , https://www.dgbestinstrument.com