Regardless of economic or environmental considerations, replacing the expensive metals that decompose exhaust gas in the catalytic converter with cheaper and more efficient materials is the first task of scientists. People need to use catalysts to perform chemical reactions, otherwise they will not be able to convert pollutant gases in car exhaust into clean compounds that can be discharged into the environment. In order to improve catalysts, researchers need to have a deeper understanding of the working principles of such catalysts.

(Image source: Stanford University)
According to foreign media reports, a team from Stanford University and the Department of Energy's SLAC National Accelerator Laboratory (Department of Energy's SLAC National Accelerator Laboratory) has accurately determined which pair of atoms in palladium and platinum nanoparticles decomposes this Class exhaust is the most active. They also answered a question that plagued catalyst researchers: Why are larger size catalyst particles sometimes better than smaller size, which is completely contrary to everyone's original idea? The answer is related to the way in which such particles change shape during the reaction, creating more highly active sites.
The results of this research are an important step towards improving the performance of catalysts in industrial processes and emission control. People need to use catalysts to carry out chemical reactions, otherwise the polluting gases in car exhaust cannot be converted into clean compounds that can be discharged into the environment. In automotive catalytic converters, nanoparticles of precious metals such as palladium and platinum are attached to the ceramic surface. When the gas to be discharged flows through, the atoms on the surface of the nanoparticles will adsorb the gas molecules, prompting them to react with oxygen to produce water, carbon dioxide and other less harmful chemicals. Each particle catalyzes billions of reactions before being exhausted.
The researchers said that current catalytic converters are designed for high-temperature operation, so most harmful emissions come from vehicles that have just begun to heat up. As more and more engines are designed to work at lower temperatures, there is an urgent need to find new catalysts that can work better under such temperature conditions. At the same time, such catalysts also need to be used in the short term. Converted to electric ships and trucks.
(Image source: Stanford University)
But what makes one catalyst more active than the other? The answer has always been elusive. In this study, the research team observed catalyst nanoparticles made of platinum and palladium from both theoretical and experimental perspectives to see if they could identify specific atomic structures on their surfaces that achieve higher activity.
In terms of theory, SLAC scientist Frank Abild-Pedersen and his research team created a new method at the SUNCAT Interface Science and Catalysis Center to simulate how exposure to gas and steam in chemical reactions affects the shape and atomic structure of catalytic nanoparticles . Abild-Pedersen said this is computationally very difficult, and previous studies assumed that the particles exist in a vacuum and will not change.
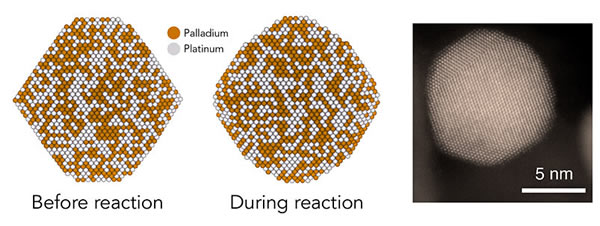
His team created simpler new methods to simulate particles in a more complex and realistic environment. The calculations of postdoctoral researchers Tej Choksi and Verena Streibel show that as the reaction proceeds, the octahedral nanoparticles will become more rounded, so that their flat surface will become a series of jagged steps.
By creating and testing nanoparticles of different sizes, each with different ratios of jagged edges and flat surfaces, the team hopes to accurately find the structural settings, even atomic settings, that have the most effect on the catalytic activity of this type of particle.
Doctoral student Angel Yan produced nanoparticles with precisely controlled sizes, each containing a uniformly distributed mixture of palladium and platinum atoms. In order to do this, new methods must be developed to produce larger particles by "seeding" around smaller particles. Yang used X-rays from the SLAC Stanford synchrotron radiation source to confirm the composition of the nanoparticles.
Yang then conducted experiments using nanoparticles of different sizes to catalyze a reaction that converts propylene (one of the most common hydrocarbons in exhaust gas) into carbon dioxide and water. Yang said: “Water plays an interesting and beneficial role here. Normally, water can poison or deactivate the catalyst, but now, contact with water makes the particles rounder and opens up more active sites. "
The experimental results confirmed that the larger particles are more active, become rounder and more obvious in the reaction, as predicted by computational research. The most active particle contains the largest proportion of the specific atomic configuration. There are 2 atoms, and each atom is surrounded by 7 adjacent atoms to form a pair for reaction. This 7-7 pair makes the larger particles larger than the smaller particles. Perform better.
In the future, Yang hopes to figure out how to "seed" nanoparticles with cheaper materials, thereby reducing costs and reducing the use of rare and precious metals. (Yu Qiuyun)
Pu Pouches,Pu Leather Drawstring Pouch,Pu Makeup Pouch,Pu Leather Drawstring Bag Gift Pouch
Dongguan C.Y. RedApple Industrial Limited , https://www.zjhpgbags.com