Pb-based and Co-based oxides have been extensively studied as important functional materials (such as ferroelectric, piezoelectric, ferromagnetic, catalytic, and battery functional materials). Surprisingly, however, no ternary compound system of Pb-Co-O has been reported yet. Analogous to the most widely studied magnetoelectric multiferroic star material, BiFeO3, PbCoO3 with an ABO3-type perovskite structure is expected to be an important multiferroic material because the lone pair electron effect of the A-site Pb ion can lead to an iron electrode. The Co ions at the B site can form long-range magnetic ordering, which is expected to achieve the coexistence of the two degrees of freedom of polarization and magnetic ordering. Among the 3d transition metal perovskite oxide PbMO3 (M = Ti, V, ..., Ni), PbCoO3 is the only material system that has not yet been successfully prepared. With the gradual increase of the number of electrons in the transition metal d, a series of interesting changes have occurred in the valence state of Pb and transition metal M in the PbMO3 system. For example, in PbTiO3 and PbVO3 where the number of d electrons is smaller, a charge configuration of Pb2+M4+O3 is formed; in PbCrO3 and PbFeO3, the average valence state of the A site Pb ion becomes +3, forming Pb3+M3+ O3 type charge combination; when M = Ni, rare Pb4+ valence appears at the A site of the perovskite, and the charge distribution of the system becomes Pb4+Ni2+O3. Correspondingly, these PbMO3 systems with different charge configurations exhibit very unique physical properties. In the periodic table, Co is between Fe and Ni, and the charge combination of the corresponding compound is just in the critical region of Pb3+M3+O3 and Pb4+M2+O3. Therefore, if the PbCoO3 perovskite can be experimentally obtained, its peculiar charge form and special physical properties are also very attractive.
Recently, the team of Ex6 researchers at the Institute of Physics, Chinese Academy of Sciences/Beijing National Laboratory for Condensed Matter Physics, and the team of Long Youwen cooperated with the team of Professor M. Azuma of Tokyo Institute of Technology to use high-temperature and high-pressure technology for the first time. The PbCoO3 system with perovskite structure was successfully prepared, and the unique charge properties and physical properties of the material were found.
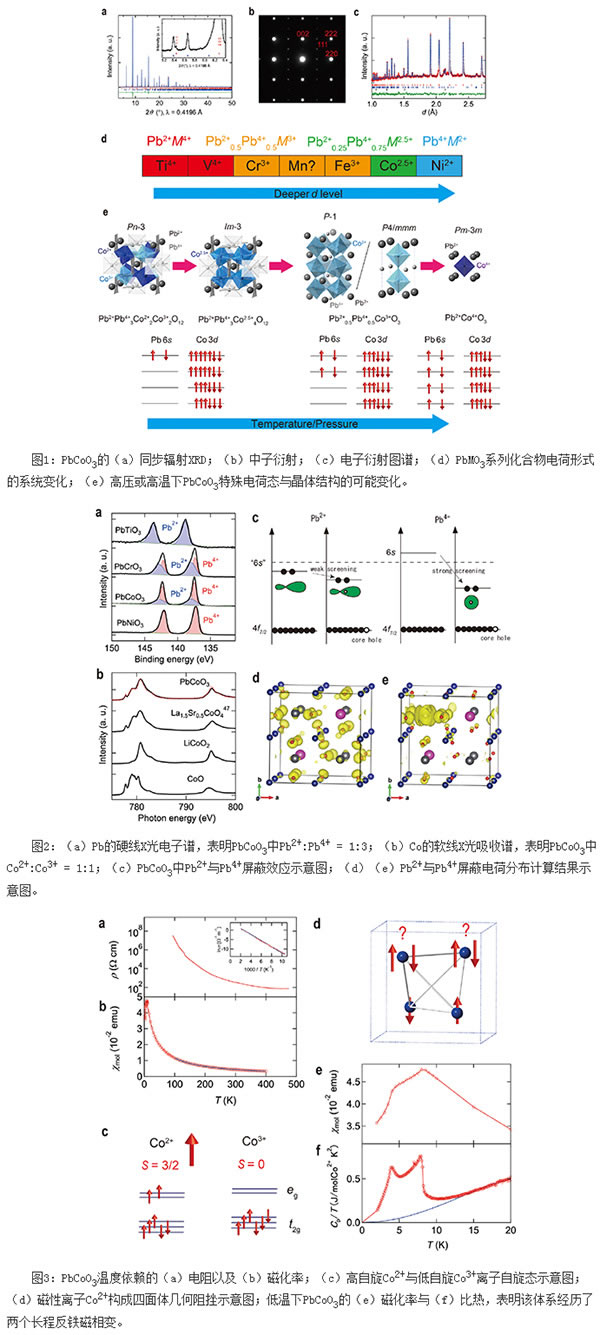
Experiments show that PbCoO3 has very strict phase formation conditions, the pressure can not be lower than 10 GPa, and the phase change temperature range does not exceed 50 K. This sensitive synthesis condition may indicate the competition of multiple charge states. In order to fully characterize the physical and chemical properties of this new system, the researchers conducted a series of studies such as synchrotron XRD, neutron diffraction, electron diffraction, X-ray absorption spectroscopy, magnetic susceptibility, magnetization, electrical resistance, specific heat, and theoretical calculations. The results show that although this compound has a simple chemical formula of PbCoO3, the Pb at the A position has a Pb2+ and Pb4+ valence of 1:3, while the Co at the B position has a 1:1 salt-rock orderly Co2+ and Co3+ valence, and Co2+ is a magnetic high spin state (S = 3/2), but Co3+ is a non-magnetic low spin state (S = 0). Therefore, taking into account the special charge distribution of the material, the chemical formula can be expressed as the A-site and the B-site at the same time the four-order perovskite Pb2+Pb34+Co22+Co23+O12, space group is cubic Pn-3. Due to the ordered distribution of the salt rock type of low-spin Co3+ ions and high-spin Co2+ ions at B-site S = 0, the material exhibits electrical semiconductor behavior consistent with the thermal activation model. Magnetically, if only magnetic Co2+ ions with S = 3/2 are considered, they will constitute a geometric hindrance of the tetrahedron. In general, this geometrically frustrating magnetic system does not have long-range magnetic order. Interestingly, however, PbCoO3 (Pb2+Pb34+Co22+Co23+O12) exhibits two long-range antiferromagnetic phase transitions at lower temperatures, with transition temperatures around 8 K and 4 K. The abnormal long-range antiferromagnetic ordering behavior in this geometrically frustrated system may be related to the slight structural distortion of the tetrahedron. Recalling the harsh synthesis conditions of PbCoO3, it is not difficult to imagine that this peculiar charge combination Pb2+Pb34+Co22+Co23+O12 is very sensitive to external conditions. If pressure is used for regulation, it is expected that the series transition of charge and structure-property phase transition under high pressure will be as follows: Pb2+Pb34+Co22+Co23+O12 (A and B sites are ordered simultaneously)→Pb2+Pb34+Co42.5 +O12 (A position 1:3 ordered)→Pb2+Pb4+Co23+O6 (A position 1:1 orderly)→Pb4+Co4+O3. So far, this work has also clarified the change in the charge form of the PbMO3 series perovskite, with increasing number of d electrons being Pb2+M4+O3 (M=Ti,V)→Pb3+M3+O3 (M=Cr,Mn, Fe)→Pb3.5+M2.5+O3 (M=Co)→Pb4+M2+O3 (M=Ni).
The results of the relevant studies were published in the recent "Journal of the American Chemical Society" (J. Am. Chem. Soc. 139, 4574-4581 (2017)). The work was supported by projects of the Ministry of Science and Technology (2014CB921500), the National Natural Science Foundation of China (11574378) and the Chinese Academy of Sciences (XDB07030300).
Construction Work Clothes,Safety Work Uniforms,Construction Protective Uniforms,Construction Worker Uniform
Xinxiang Worldbest Patron Saint Co., Ltd , https://www.xxhyhsworkwear.com