 |
Researchers at the SLAC National Accelerator Laboratory and Stanford University of the Department of Energy have demonstrated for the first time that an inexpensive catalyst can decompose water for hours in commercial equipment and harsh environments And produce hydrogen.
Electrolyzer technology, based on polymer electrolyte membranes (PEM), is likely to use renewable energy to produce hydrogen on a large scale, but high-cost precious metal catalysts such as platinum and iridium have been hindering its development.
The researchers reported in the journal Nature-Nanotechnology today that the research pointed the way to finding a cheaper catalyst solution.
(Photo from: Greg Stewart / SLAC National Accelerator Laboratory)
"Hydrogen is a very important industrial chemical that can be used to make products such as fuels and fertilizers," said Thomas Jaramillo, director of the SUNCAT Interface Science and Catalysis Center who leads the research team. "It is also a clean, high-energy molecule that can be used in fuel cells or to store energy produced by variable energy sources such as solar and wind energy. But most of the hydrogen produced today is made from fossil fuels, increasing carbon dioxide in the atmosphere Level. We need a low-cost way to produce with clean energy. "
From expensive metals to cheap and abundant materials
For many years, people have been developing alternatives to precious metal catalysts for PEM systems. Many materials that have been proven to work in a laboratory environment have been discovered, but Jaramillo said that to the best of his knowledge, this is the first time high performance has been demonstrated in a commercial electrolyzer. The equipment is produced by the PEM Electrolysis Research Base in Connecticut and produced by Nel Hydrogen, the world's oldest and largest manufacturer of electrolysis equipment.
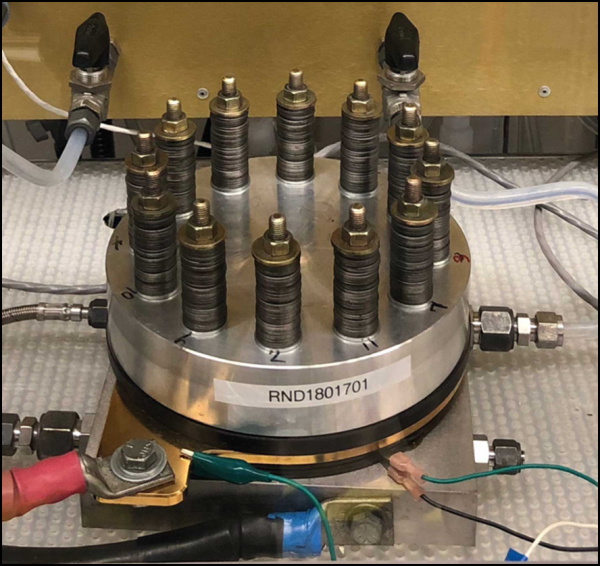
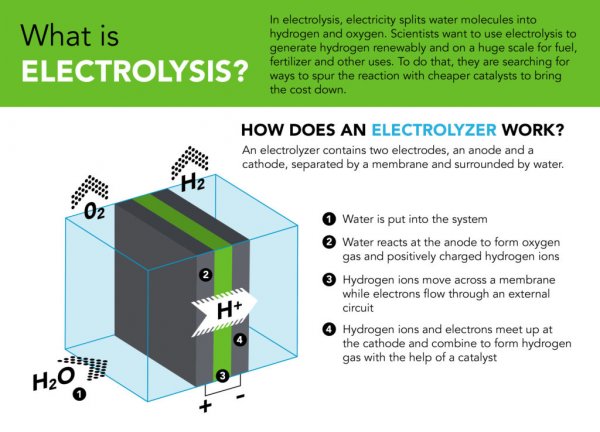
Industrial electrolytic cell used in the experiment. Electrodes sprayed with catalyst powder are stacked in the central metal plate and compressed and fixed with bolts and washers. Water flows in through the tube on the right, and hydrogen and oxygen are discharged through the tube on the left. (Picture from: Nel Hydrogen)
The working principle of electrolysis is much like the reverse process of a battery: it does not generate electricity, but uses electricity to split water into hydrogen and oxygen. The reactions that produce hydrogen and oxygen are carried out on different electrodes using different precious metal catalysts. In this case, the Nel Hydrogen team replaced the platinum catalyst on the hydrogen-producing side with a catalyst composed of cobalt phosphide nanoparticles deposited on carbon to form a fine black powder, which was studied by SLAC and Stanford University. Personnel manufacturing. Just like other catalysts, it brings other chemicals together and causes them to react.
The cobalt phosphide catalyst performed very well throughout the test process (more than 1,700 hours), indicating that it was sufficient for daily reactions. The experiment was led by SUNCAT research engineer Laurie King.
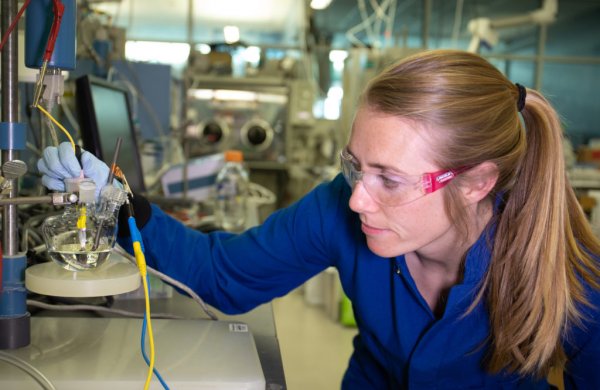
The equipment used by Stanford graduate student McKenzie Hubert can test cheap catalysts in the laboratory. The team led by SLAC and Thomas Jaramillo, director of the Stanford University ’s SUNCAT Center, demonstrated for the first time that this inexpensive material can achieve high performance in commercial electrolyzers. (Photo from: Jacqueline Orrell / SLAC National Accelerator Laboratory)
"Our team has been studying this catalyst and related materials for some time," Hubert said, "and we start from the experimental stage of the basic laboratory scale, by testing it under industrial operating conditions, under which you It needs to cover a larger surface area with a catalyst, and it must function under more challenging conditions. "
One of the most important elements of this research is to expand the production of cobalt phosphide catalyst while keeping it very uniform-the process involves the synthesis of raw materials on a laboratory bench, grinding with a mortar and pestle, baking in an oven and Finally, the very fine black powder is turned into ink, and then sprayed on porous carbon paper. The resulting large-format electrode was loaded into an electrolytic cell for hydrogen production test.
Mass production of hydrogen
Although the development of the electrolytic cell was funded by the Department of Defense, which is very interested in the generation of oxygen by electrolysis of submarines, Jaramillo said that this work is also in line with the US Department of Energy ’s H2 @ Scale program, which brought DOE experiments. The joint efforts of laboratories and industry have promoted the trend of hydrogen to produce, transport, store, and use hydrogen that is affordable in many applications. Fundamental catalyst research was funded by the US Department of Energy ’s Office of Science.

(Photo from: Greg Stewart / SLAC National Accelerator Laboratory)
Katherine Ayers, vice president of research and development at Nel and co-author of the paper, said: "The collaboration gives us the opportunity to understand whether these catalysts can be stable in the long term and provides us with an opportunity to understand whether they can provide better results than platinum Performance. "
She said: "The performance of the cobalt phosphide catalyst needs to be improved, and the scale of its synthesis must be expanded. But I was surprised by the stability of these materials. Although their hydrogen generation efficiency is still lower than platinum, it is constant. In this environment, many factors may cause degradation. "
Ayers said that although platinum catalysts only occupy about 8% of the total cost of hydrogen produced by PEM, the fact is that the precious metals market is so volatile and prices continue to fluctuate, which may hinder the development of the technology. With the improvement of other aspects of PEM electrolysis technology and the increasing demand for hydrogen, reducing and stabilizing the cost of catalysts will become increasingly important.
Source: "Natural Nanotechnology, October 14, 2019" (10.1038 / s41565-019-0550-7)
(Original source: Fuel Cell Engineering China New Energy Network Comprehensive)
Pressure test box
The pressure test box, composed of pressure hose, pressure tap and Pressure Gauge, is used for the pressure testing of the hydraulic system. It is has simple use and good portability. The pressure hose is used for the the pressure testing of the hydraulic system under the operating pressure of 630BAR and burst pressure of1600BAR, temperature-30~+100℃. It can read the pressure value directly with connection to the pressure gauge. It is wildly used in the military, mining, ship, oil and chemical industry.
Pressure Test Box,Metal Test Box For Pressure Check,High Pressure Accelerated Aging Test Box,Pressure Accelerated Aging Test Box
wuxi kaifeng pressure gauge co., ltd , https://www.wxkfmanometer.com